There is little doubt when a water supply contains very much iron or manganese because of the brown to black stains which these minerals cause. The stains show up rapidly in sinks, and appear on laundered fabrics and every surface touched by the water. The Safe Drinking Water Act of 1974 provided a recommended Secondary Drinking Water Regulation which suggests limits of 0.3 mg/L (parts per million) of iron and 0.05 mg/L (ppm) of manganese because of the stains which may be caused by higher concentrations.
Iron and manganese produce unpleasant tastes when present in high concentrations, and can affect both flavor and color of foods. These impurities react with the tannins present in coffee, tea and some alcoholic beverages to produce a black sludge which affects both taste and appearance. An early form of ink was made by mixing iron salts with tannins. In nature, iron is found as several different oxides, carbonates and sulfides, and it is found as traces in many different minerals. Although man reduces the various iron ores to metallic iron, the metal has a strong tendency to revert to the more stable oxide forms. Iron exists in three states, which are related as follows:

Deep in the earth, far from the oxidizing effects of oxygen in the air, conditions favor the reduction of the natural ferric iron deposits to the ferrous state. Since the ferrous iron is quite soluble, it is readily dissolved and carried in groundwater, in clear and colorless solution. However, when this water is brought to the surface and exposed to oxygen in the air, it is rapidly converted back to the ferric state. The ferric iron then reacts with alkalinity in the water to form ferric hydroxide, the insoluble brown precipitate which causes so much staining. Similar reaction occur with manganese, although they are slower than with iron. Household bleaches can oxidize iron and manganese to the insoluble states, causing the staining problems when bleaches are used in laundering.
The corrosion of iron or steel water lines may also add iron to water. The partial oxidation of metallic iron to the ferrous state allows the iron to dissolve in the water, and to be carried through the water system. However, if oxygen is present or oxidizing agents are added, the insoluble ferric hydroxide id readily formed. This too may be carried through the water system, or may settle in the water lines during periods of low flow, to be stirred up and carried to the point of use during high water usage.
Iron and manganese may also be present in water in combination with organic matter. Many natural and man-made organic compounds will react with iron and manganese to form heavily colored materials which can cause severe staining. These materials are usually very stable, and resist breakdown and removal.
Iron bacteria and manganese bacteria are two special forms of organic growths sometimes found in water. Although they do not cause disease, these organisms are capable of using iron and manganese in their metabolism, and may even attack steel pipe to obtain iron. As the bacteria grow, they form masses of gelatinous and filamentous organic matter which trap the iron and manganese they use. Heavy growths can completely plug pipes, but usually break away during periods of high water flow to produce “slugs” of dirty, iron or manganese-laden water, with obnoxious tastes and odors. A brown, slime-like growth in a toilet flush tank is a good indication of the presence of such organisms in a water system.
Removal: Water Softeners & Iron Removal Filters
When low to moderate amounts of dissolved iron or manganese are present in water, household water softeners will remove these impurities with the hardness in concentrations determined by the softener manufacturer. The soluble iron and manganese are removed from the water by ion exchange, just as with hardness, and when the water softener is regenerated, the iron, manganese, and hardness will be flushed down the drain.
If some of the iron or manganese is precipitated, it will be filtered out of the water by a softener. However, the gelatinous precipitate tends to stick to the water softening media, gradually building up in the bed. Backwashing the softener usually removes most of the precipitated iron, but some may remain behind. In time, this residue can coat the softening material, gradually reducing the ability of the softener to remove the hardness.
Where the fouling is light, the water softening media can be cleaned periodically with one of the commercial products made for this purpose. Some of these products are also designed to be mixed with the salt so that a mild cleaning occurs with every regeneration. Special salts are also available in which the cleaning materials are blended directly with the salt, so that no other additives are necessary, and the softener is treated with every regeneration.
Where the iron or manganese concentration is too high for a water softener, where much of the iron is already precipitated, or if only iron removal without softening is desirable, iron removal filters are often used. The media in these filters is capable of oxidizing dissolved iron or manganese to the insoluble state and removing the precipitated matter – all in the same tank. These filters must be backwashed periodically to flush the accumulated deposits down the drain, and “regenerated” with potassium permanganate to restore the oxidizing power of the filter media. These iron filters are effective up to reasonably high concentrations of iron and manganese.
Removal: Chlorination & Carbon Filtration
Neither water softeners nor the iron filters will be effective if the iron or manganese is bound into organic matter, if iron or manganese bacteria are present, or if the iron or manganese concentrations are really high. Iron or manganese bound into organic compounds is not available for removal by ion exchange, and the oxidizing power of the iron filter media is not strong enough to break these materials down. Thus, organic iron or manganese compounds often pass through softeners and filters with no significant removal. Similarly, iron or manganese bacteria can foul softeners and filters rapidly, and even when removal is good part of the time, high flow rates may cause “slugs” of dirty water to appear in the treated water.
In such cases, a chemical feed pump can be used to introduce a solution of household hypochlorite bleach into the water system ahead of the pressure tank. The chemical feed pump can be wired to operate with the well pump, thus giving good proportioning of the bleach to the water. This produces disinfection of the water with oxidation of the iron and organic matter.
Just as in disinfection, the feed rate of the chlorine is usually adjusted to produce a chlorine residual of 3 to 5 ppm at the outlet of the pressure tank. This will cause the oxidation of the iron, manganese, and organic matter usually in the time it takes for the water to flow through the pressure tank. In a few cases, additional contact time is necessary to obtain complete destruction of the organic matter present. An activated carbon filter in the water line from the pressure tank may then be used to remove the precipitated matter and excess chlorine from the water. This filter must be backwashed periodically to flush the accumulated solids to a drain, and small amounts of activated carbon added from time to time to replace that consumed by the chlorine. Since the chlorine solutions tend to lose strength, they should be made up fresh each week.
An additional advantage of this process is that it can be used to neutralize acid waters. When the pH of the water is low, it can be very corrosive. Complete removal of iron and manganese will not always be obtained if the pH is below about 7.5. Where such conditions exist, common soda ash solution may be used to neutralize the acidity present and to increase the pH to 7.5 to 8.0. Thus, the water will be made less corrosive, and good iron and manganese removal will be achieved.
Further, the plumbing in the home can be arranged to provide hard, iron-free water for sprinkling and toilets where the hardness is not important, and a softener installed to provide softened water for other water lines in the home.
Removal: Sequestration By Chemical Feeding
Another process which is available for the treatment of water containing dissolved iron and manganese is known as sequestration. Food grade polyphosphate compounds are fed into the water by a chemical feeder or a chemical feed pump, and these compounds react chemically with the dissolved iron and manganese ions to keep them in solution and thus prevent problems caused by staining and deposition in pipes and water-using equipment.
Chemical feeders are generally of a tank-type design utilizing one or both of the feed-rate control factors of solubility of the chemical compound and flow rate through the dispenser. For a greater degree of feed-rate control, some chemical feeders incorporate pressure differential devices and/or precision orifices or regulator valves within the feeder itself or installed in the water line. The polyphosphate compound in liquid solution can also be added to the water by means of a chemical feed pump which can be set to inject a specific amount of the solution into the water at regular intervals.
NOTE: The determination of which treatment method is best should be made only after careful consideration of many factors such as economics, water quality characteristics, the end use to which the water is to be put, temperature variances of the water to be treated, the inherent limitations of the available treatment technology, and others. This determination can best be made by your local water treatment representatives and they should be consulted prior to the purchase and installation of any water treatment equipment.
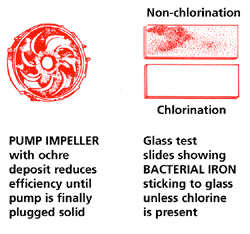
Chlorination: Bacterial Iron
All elements come together in the well where the bacteria precipitates the iron and a bio fouling mass called OCHRE is formed – a sticky, slimy deposit that clogs and plugs water systems. The slime producing BACTERIA can convert sulfates in water to corrosive hydrogen sulfide gas or a musty smell. Because of the biological action, water from the well can contain more iron than found in ground water.
Further Research
Tests conducted by the University of Florida indicate that there are many types of iron precipitating bacteria that combine with iron and nutrients in natural ground water and form a bio fouling mass called OCHRE. While the ochre and bacteria are harmless to humans, it plugs wells and water systems. Further tests show that eliminating the bacteria by chlorination prevents the formation of ochre and the iron that is precipitated is a non-sticky, porous substance that DOES NOT deposit on water system surfaces.
Benefits & Features
- Eliminates bacterial iron at its source
- Dissolves ochre iron deposits
- Eliminates the growth of the bio fouling mass ochre
- Deep well chlorination treats and protects the entire water system continuously
- Easiest user-operable chlorinator available
- Uses dry chlorine pellets
- Eliminates hydrogen sulfide algae, slime and nuisance bacteria
- First step treatment to total iron removal
- EPA registered pellets approved for drinking water
- Fully adjustable